
PharmaShots Weekly Snapshots (September 04–08, 2023)
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, M&A, DigiHealth, MedTech and Animal Health. Check out our full report below:
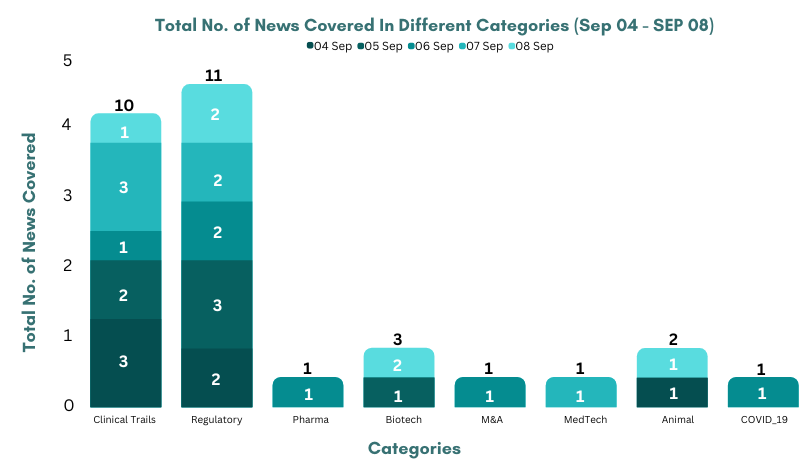

- Alnylam highlighted P-II study (KARDIA-1) results of Zilebesiran for hypertension in patients at high cardiovascular risk showed a dose-dependent, clinical reduction in 24hr. mean systolic blood pressure
Read more: Alnylam
- Arcutis highlighted interim results from the P-III (INTEGUMENT-OLE) trial of Roflumilast for mild to moderate atopic dermatitis, were found to be well-tolerated with durable & improved efficacy over time
Read more: Arcutis
- Roche highlighted P-III (ALINA) study results of Alecensa (alectinib) for ALK-positive early-stage lung cancer & showed a significant and clinical improvement in DFS
Read more: Roche
- Rallybio highlighted P-I single ascending dose results of RLYB116 for complement-mediated diseases, presented at ICW 2023 showed a reduction in free C5 ≤99% at 24hrs.
Read more: Rallybio
- Valneva and Pfizer highlighted P-II study (VLA15-221) results of VLA15 for Lyme Disease showed a strong anamnestic Ab response for all serotypes in pediatric, adolescent & adults
Read more: Valneva and Pfizer
- Janssen highlighted P-III study (MARIPOSA-2) results of Rybrevant (amivantamab-vmjw) for EGFR-mutated NSCLC showed a significant and clinical PFS improvement
Read more: Janssen
- Brii Biosciences highlighted interim results from the P-II study of BRII-179 (VBI-2601) + PEG-IFNα for Chronic Hepatitis B
Read more: Brii Biosciences
- invIOs starts the patient enrolment of APN401 in the P-Ib trial (PALINDROM) for solid tumors, conducted in Austria with 2 GMP-compliant manufacturing sites in Vienna and Linz
Read more: invIOs
- Genmab and Seagen highlighted P-III trial (innovaTV 301) results of Tivdak (tisotumab vedotin-tftv) for recurrent or metastatic cervical cancer which met its 1EPs of OS
Read more: Genmab and Seagen
- Oncorena treated first renal cancer patient with ONC175 (orellanine) in the P-I/II trial
Read more: Oncorena

- The NMPA has approved AstraZeneca’s Calquence (acalabrutinib) for chronic lymphocytic leukaemia in China, based on the P-III (ASCEND) and P-I/II trials
Read more: AstraZeneca
- The NMPA has approved Gloria Biosciences’ Zimberelimab for recurrent or metastatic cervical cancer in China, based on the P-II clinical trial results
Read more: Gloria Biosciences
- NICE has recommended BMS’ Camzyos (mavacamten) as an add-on treatment option for adults with symptomatic obstructive hypertrophic cardiomyopathy
Read more: BMS
- The MHLW has approved Guardant Health’ Guardant360 CDx as companion diagnostic for Enhertu to treat non-small cell lung cancer
Read more: Guardant Health
- The US FDA has accepted the BLA of Genentech’s Crovalimab for Paroxysmal Nocturnal Hemoglobinuria, based on the P-III trial (COMMODORE 2)
Read more: Genentech
- The NICE has recommended Chiesi’s Elfabrio (pegunigalsidase alfa) for adults with Fabry Disease, based on results from P-III clinical trials
Read more: Chiesi
- The MHRA has granted marketing authorisation of AbbVie’ Aquipta (atogepant) for the Prevention of migraines in adults, based on (ADVANCE) trial for episodic and (PROGRESS) for chronic migraine
Read more: AbbVie
- Italfarmaco Group received EMA’s validation of MAA for Givinostat to treat duchenne muscular dystrophy, based on the P-III trial (EPIDYS) results
Read more: Italfarmaco Group
- The US FDA has granted the BTD to INOVIO’ INO-3107 for recurrent respiratory papillomatosis, based on the P-I/II trial results showed 81.3% of patients had a decrease in surgical interventions
Read more: INOVIO
- Janssen submit MAA to the EMA for Erdafitinib in LA or metastatic urothelial cancer with susceptible FGFR alterations, based on the results from Cohort 1 of the P-III study (THOR)
Read more: Janssen
- The US FDA has granted IND approval for P-II trial (VB-C-04) to evaluate Nykode Therapeutics’ VB10.16 in HPV16-positive cervical cancer
Read more: Nykode Therapeutics

- Braeburn’s Brixadi (buprenorphine) extended-release injection is now available in the US for moderate to severe opioid use disorder
Read more: Braeburn

- Arcturus Therapeutics and CSL announced EMA’s validation of MAA for ARCT-154 vaccine to prevent COVID-19
Read more: Arcturus Therapeutics and CSL

- The US FDA has approved Boston Scientific’ WATCHMAN FLX Pro Left Atrial Appendage Closure Device to reduce stroke risk in patients with NVAF who need an alternative to oral anticoagulation therapy
Read more: Boston Scientific

- Nurix & Seagen collaborated on new class of cancer therapies & Seagen will be responsible for conjugating the selected degraders to antibodies, turning them into degrader-antibody conjugates
Read more: Nurix & Seagen
- Otsuka collaborated with ShapeTX to develop novel AAV gene therapies for ocular diseases combining ShapeTX’s AAVid capsid discovery platform and transgene engineering technology with Otsuka’s expertise in genetic payload design and ophthalmology
Read more: Otsuka and ShapeTX
- Zenas BioPharma & BMS collaborated to develop and commercialize Obexelimab for autoimmune diseases in Japan, South Korea, Taiwan, Singapore, Hong Kong and Australia
Read more: Zenas BioPharma & BMS

- Abbott to acquire Bigfoot Biomedical to make diabetes management more precise & personal
Read more: Abbott and Bigfoot Biomedical

- Novozymes & Bactolife collaborated to finish the development and commercialization of biosolution Ablacto+ to reduce post-weaning diarrhea & antibiotic consumption
Read more: Novozymes & Bactolife
- Elanco launch Varenzin-CA1, the first-of-its-kind oral treatment for Anemia in Cats with CKD in the US
Read more: Elanco
Related Post: PharmaShots Weekly Snapshots (August 28 – September 01, 2023)
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














